Primary Intramedullary Meningeal Melanocytoma in Cervical Spine: A Case Report and Literature Review
Article information
Abstract
Primary intramedullary meningeal melanocytoma is an extremely rare tumor that occurs in the spinal cord. To our knowledge, 29 cases have been reported. We report a case of intramedullary meningeal melanocytoma with a narrative literature review: a 78-year-old man with the chief complaint of right-side weakness over the course of six months. Magnetic resonance imaging revealed an intradural intramedullary tumor between C3 and C5 levels that showed hypo-intensity on T2 and mild homogenous hyper-intensity on gadolinium-enhanced T1-weighted images. Myelotomy was performed for tumor removal, and a black-pigmented tumor was observed under a surgical microscope. Histopathological examination confirmed intramedullary meningeal melanocytoma. Since radiologic findings are insufficient to diagnose primary intramedullary meningeal melanocytoma, histopathological examination must be performed for diagnosis. Complete tumor resection is the most important prognosis, and adjuvant radiotherapy should be considered to prevent malignant transformation and recurrence.
INTRODUCTION
Intramedullary meningeal melanocytoma is an exceptionally rare tumor that occurs in the central nervous system (CNS). Meningeal melanocytoma was first described in 1972 by Limas and Tio17), defined as a pigmented tumor of the leptomeninges. Intramedullary meningeal melanocytoma is known to be derived from melanocytes located in leptomeninges3,29). During the embryologic period, melanocytes travel from leptomeninges to the spine, and cellular proliferation affects the characteristics of the cell to become a tumor. From the database of PubMed and Google Scholar, a total of 29 cases of intramedullary meningeal melanocytoma were found between 1970 and 2020. Here, we report a case of intramedullary meningeal melanocytoma in the spinal cord and provide an overview of the available literature. Additionally, this is the first case report of the same in Korea.
CASE REPORT
A 78-year-old man was admitted to our institution with the chief complaint of severe low back pain for 30 years and progressive right-side weakness over the course of six months. No remarkable past medico-surgical history was found. On physical examination, the patient suffered from low back pain around the area of L2 to L4. Mild direct tenderness was observed. On neurologic examination, the straight leg raising test was positive for both legs: 30° and 70° for the right and left legs, respectively. There was no posterior neck pain or direct tenderness on the neck, but the patient occasionally dropped an object against his will. In addition, gait disturbance was observed. The right-side motor weakness was present, and the general motor grade was between II and III on the right side. The patient suffered from right-sided numbness.
No remarkable abnormalities were found in the plane cervical X-ray images, except for degenerative disc space narrowing lesions. Computed tomography (CT) images revealed a hyperdense mass between C3 and C5 levels. The center of mass was heterogeneous. A spinal cord tumor was suspected from the images.
Magnetic resonance imaging (MRI) of the cervical and lumbar spine showed cervical intradural-intramedullary tumor between C3 and C5 levels and epidural abscess between L3 and S1 levels. The cervical lesion showed hypo-intensity on T2 and hyper-intensity on T1-weighted images (WI). Diffuse mild enhancement was revealed in gadolinium-enhanced T1-WI (Fig. 1). MRI also showed a 21 mm mass with hemorrhagic changes containing multiple septa in the center. The mass was skewed toward the right from the center of the spine. Based on T2-WI, it was suspected to be edematous and contained high vascularity. The initial impressions based on the cervical MRI findings were cavernous malformation, arteriovenous malformation, meningioma, and lymphoma.
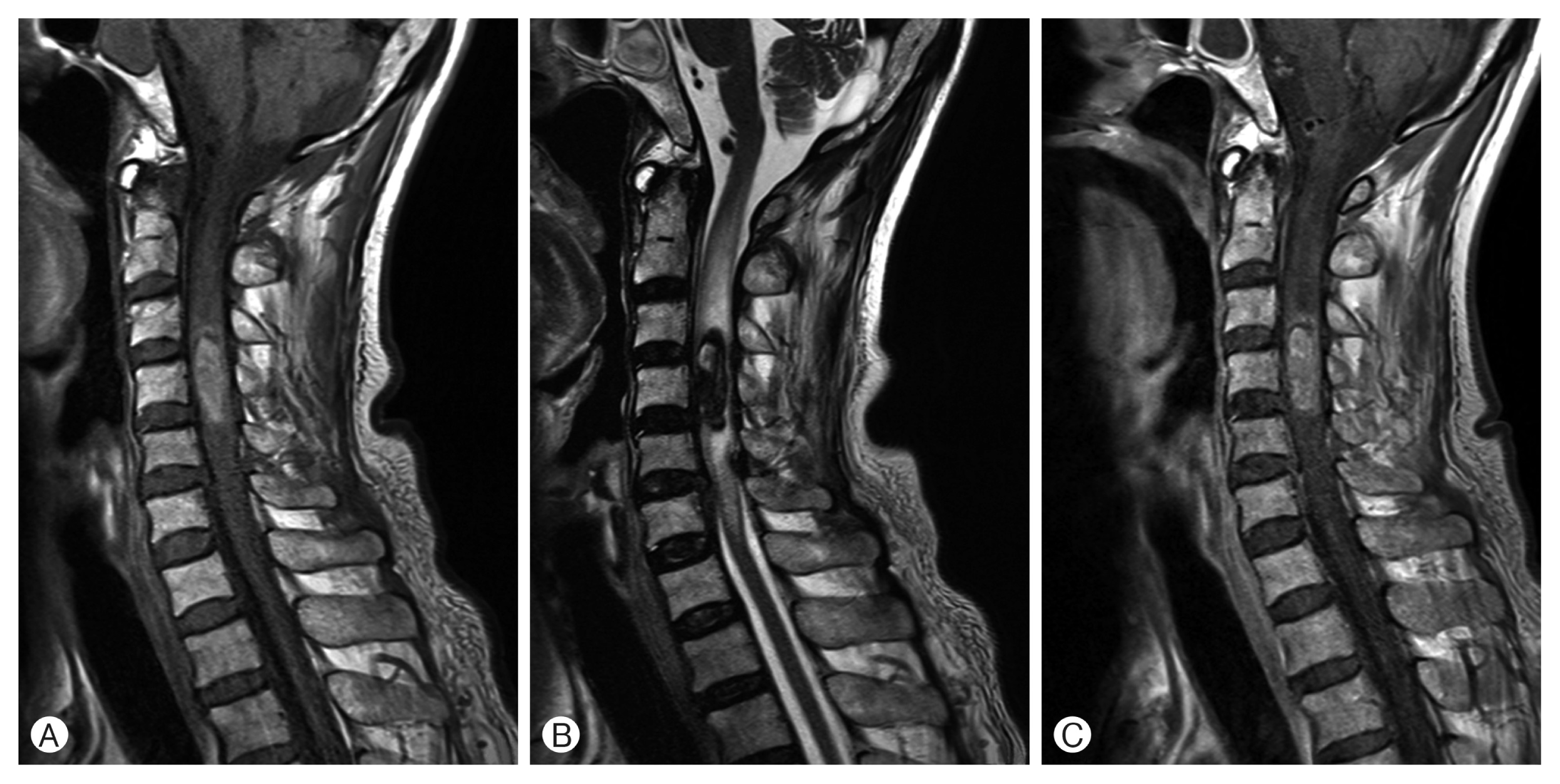
Sagittal image of magnetic resonance imaging between C3 and C5 levels before the operation. (A) T1-weighted images (WI) slightly show hyperintensity. (B) Hypointensed lesion can be observed in T2-WI with multiple septa in center. (C) Gadolinium-enhanced the mass slightly, which is revealed in the enhanced T1-WI.
Abnormal enhancement was observed on lumbar MRI in the vertebrae, intervertebral discs, and ventral epidural space between L4 and S1 levels (Fig. 2). The results suggested infectious spondylodiscitis and multiple infected cysts in the right facet joints between L4 and S1 levels. The infection was confirmed comprehensively, and ceftriaxone and vancomycin were introduced to the patient for conservative treatment.

Sagittal image of magnetic resonance imaging between L1 and L5 levels. (A) T1-weighted images (WI) show hypo-intensity on L4/5 and S1 vertebral body and intervertebral disc spaces. (B) Heterogeneous hypointensed lesions can be observed in T2-WI. (C) Gadolinium-enhanced the lesion, which is revealed in the enhanced T1-WI.
Surgical intervention was performed for the cervical lesion. During the operation, laminectomy was performed between C3 and C5 levels. After opening the dura mater under a surgical microscope, a normal colored spinal cord was noted. The surface of the right spinal cord felt harder than the left spinal cord at the C4 level. After opening the pia mater and meticulous spinal cord dissection in the midline, a black pigmented tumor mass was exposed (Fig. 3). The mass had a rubbery hard consistency and was surrounded by soft vascularized tissue. However, there was no hemorrhagic evidence, such as old blood clots. The demarcation of the tumor from the normal spinal cord was good in color, but it was not easy to find a cleavage plane between the tumor mass and surrounding normal spinal cord parenchyma. With meticulous dissection using a dissector and internal decompression with an ultrasonic aspirator, the tumor was completely removed (Fig. 3). During the procedure, intraoperative neuro-monitoring was performed. Initially, motor evoked potentials (MEP) showed a 70% decrease in the right upper extremity compared to the left. During the procedure of tumor removal, the MEP wave changed to a flat condition and remained the same by the end of surgery.

Intramedullary meningeal melanocytoma images under surgical microscope. (A) There is no sign of pigmented tumor in the subdural space. (B) Solid and vascularized mass can be seen located in the intramedullary space. (C) The pigmented mass, completely removed from the spinal cord.
Immediately after the operation, the right-side general motor was recorded to be grade I. The patient suffered from post-operative paralytic ileus. A Levin tube was inserted to decrease the abdominal pressure. Progressively, the patient recovered. Antibiotics were retained during the admission period. However, the right-sided hemiparesis did not show any improvement.
Microscopic analysis was performed for pathological diagnosis. Morphologically, there was a little activity of mitosis and no severe dysplasia. The cells were immunochemically stained with Melan-A and S-100 proteins. CD-68 was positive, and the percentage of Ki-67 was less than 5%. The official result was a benign tumor that was suitable for melanocytoma (Fig. 4).

(A) Hematoxylin and eosin stain (H&E), 5-folded magnification; scan power view shows black pigmented lesion associated with hemorrhage. (B) H&E, 30-folded magnification; on high power magnification, pigmented spindle tumor cells and heavily pigmented macrophages can be seen. Note that the oval nuclei of tumor cells lack cytological atypia. (C) Melan-A stain; tumor cells were positive for melan-A immunohistochemistry. (D) S-100 stain; tumor cells were negative for S-100 immunohistochemistry.
A proton emission tomography (PET) scan was performed to search for a primary malignant lesion. Only mild F18-fluorodeoxyglucose (FDG) uptake was observed in the spinal cord between C3 and C5 levels without a clearly defined margin, which indicates low metabolic tumor or focal inflammatory process. There was no sign of distant metastasis. MRI was performed on the second day of the operation, and it confirmed the complete resection of the tumor (Fig. 5).
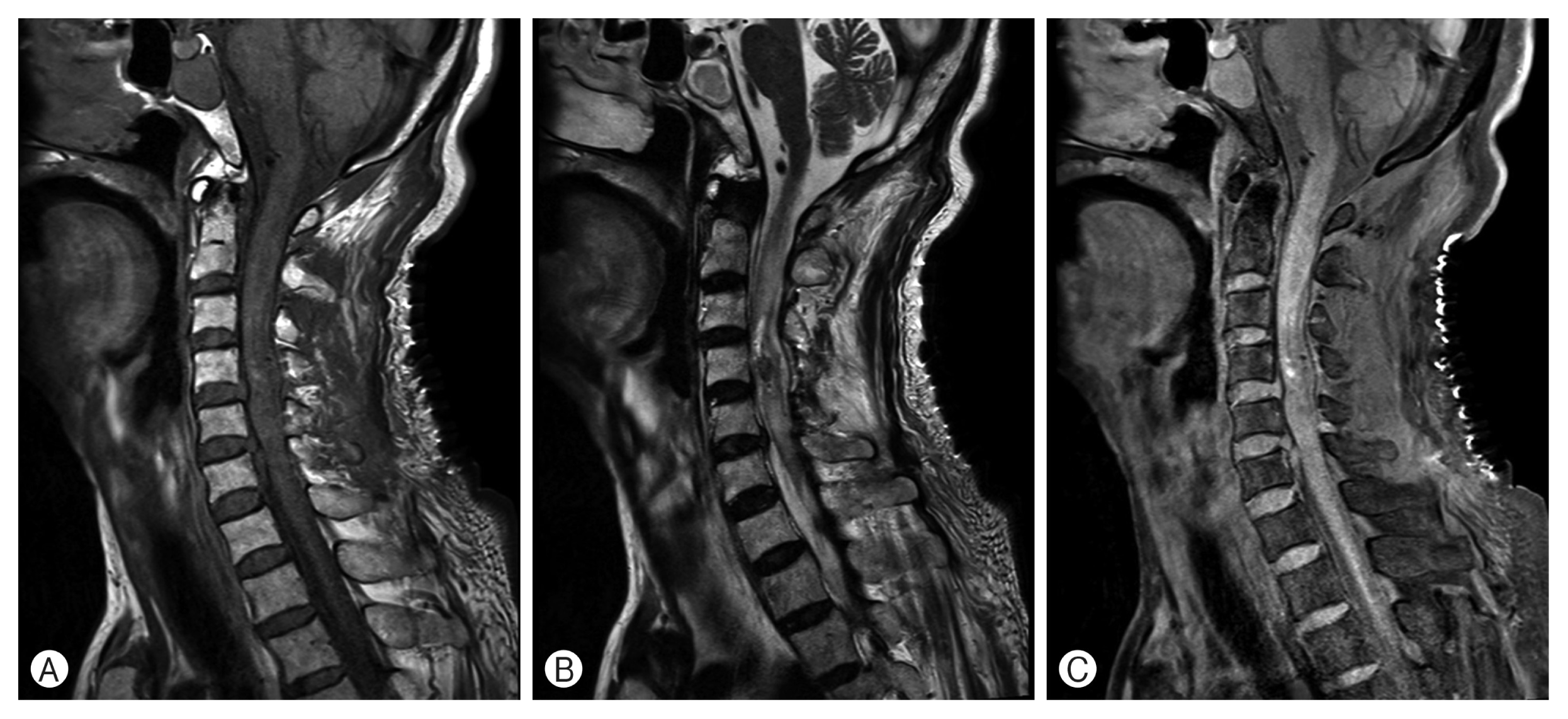
Sagittal image of magnetic resonance imaging between C3 and C5 levels after the operation. (A) T1-weighted images show no sign of remaining mass on the cervical levels. (B) The hypointensed lesion revealed in the preoperative images disappeared after the surgery. (C) No sign of definitely enhanced mass in the cervical levels.
DISCUSSION
1. Characteristics
The authors described the tumor as solitary and low-grade neoplasm with the characteristic of invading surrounding structures17). In general, meningeal melanocytoma is usually located in the intradural extramedullary compartment and is adhesive to the meninges. Intramedullary meningeal melanocytoma in the spinal cord is known to be an extremely rare tumor. The previously reported cases are summarized in Table 1. There is no large volume of analyzed research due to its low incidence rate. However, it can be presumed from the previously reported cases that melanocytoma of the spinal cord occurs most often in the thoracic levels (n=19) (Table 2).
2. Embryology
The epidermal melanoblasts reach leptomeninges during the embryogenic period of formation of the neural crest9). The neural crest undergoes differentiation to become numerous cell types, including the leptomeninges, glial cells, adrenal medullary cells, and melanocytes28). The lateral margin of the thin layer from the neural crest forms the leptomeninges after embryogenesis. Melanocytes become atypical cells that move toward malignancy with cellular differentiation and proliferation. In vitro experiments have revealed that melanocyte cells become immature ancestral cells after exposure to high concentrations of endothelin-3 substance7). In vivo, this phenomenon might be a clue toward understanding the pathogenesis of melanocyte18). Additionally, the tumor suppressor genes and proteins, CDKN2A, p16INKA, p19ARF, and PTEN, play a critical role in transformation21).
Melanocytoma is classified according to the World Health Organization classification under the subclass of melanocytic lesions of the CNS. The subclass includes other melanocytic tumors such as melanocytosis, melanomatosis, and malignant melanoma6). Most cases of meningeal melanocytoma within the spinal cord reported in the literature have been located in the intradural extramedullary compartment15). Incidence of intramedullary melanocytoma within the spinal cord is relatively rare compared to that of extramedullary melanocytoma. A total of 29 cases of intramedullary meningeal melanocytoma were found in the public databases, PubMed and Google Scholar.
3. Diagnosis
The majority of patients from the reported cases were admitted to hospitals with symptoms of motor weakness, myelopathy, and/or sensory changes. Clinicians are favoring MRI to diagnose the disease. In common, intramedullary meningeal melanocytoma appears as iso-intensity to mild hyperintensity on T1-WI and iso-intensity to hypo-intensity on T2. Gadolinium contrast slightly enhances the lesion homogenously20). The specificity of MRI for the disease entity of melanocytoma is not sufficient to diagnose the disease alone. In our case, the impression from the MRI images was cavernous malformation, not melanocytoma.
F18-FDG PET/CT is a great tool for the differential diagnosis of malignancy in addition to MRI. Primary melanoma and melanocytoma show similar features on MRI. However, biochemically, malignant tumors increase the activity of glucose transporter proteins and glucose phosphorylating hexokinase, which can be detected by F18-FDG PET/CT33).
Pathologic confirmation is the most important step in diagnosing the disease. The key to diagnosis of the disease is associated with melanin in the cells. Melanocytic tumors such as melanoma and melanocytoma are occasionally unpigmented. However, they can be diagnosed under an electron microscope or using immunohistochemical staining techniques. The tumors showed oval or spindle shape with melanin content. The distinct histological features are positive reactions to anti-melanoma antibody (HMB-45) and S-100 but negative for epithelial membrane antigen18). Cellular morphology is considered to be a prognostic factor. The spindle-shaped morphology shows a relatively better prognosis than oval-shaped19). The difference between melanoma and melanocytoma is the degree of abnormal mitosis and atypical cytology. Melanocytoma generally shows an absence or a low-grade atypical cytology18). In our case, the tumor showed a spindle-shaped melanin content with a low grade of mitosis (Ki-67 < 5%) and no severe dysplasia under the microscope.
Immunohistochemical markers are not useful for distinguishing melanocytic tumors due to the almost identical reactivity to HMB-45 and S-10023). Molecular analyses have recently been highlighted to solve this problem. Melanocytoma shows no mutation in the G α q (GNAQ) gene on the long arm of chromosome 9), but melanoma does. The GNAQ gene encodes oncoprotein that activates the mitogen activation protein kinase pathway related to malignancy. Hoffmann et al.11) used molecular analyses of GNAQ and avoided the misdiagnosis of malignant melanoma instead of melanocytomas. It is also useful to determine whether the lesion primarily originates from the CNS. GNAQ and guanine nucleotide-binding protein subunit α-11 (GNA11) gene mutations are distinct features of the CNS melanocytic tumors23). Molecular analyses were recommended to the patient for diagnosis with a cost, but the patient refused to do so because of the poor financial status.
4. Treatment
No consensus was made with the first-line treatment for the tumor. However, many case reports have recommended its complete resection. Melanocytoma is generally considered a benign disease but has the potential to recur and become malignant11,18,25).
Adjuvant radiotherapy is also emphasized by many authors for the reasons mentioned above. Rades et al. 26) reported a prognostic analysis of intracranial and intraspinal meningeal melanocytomas. The patients who underwent complete resection showed 100% 5-year survival rates regardless of radiotherapy. However, incomplete resection showed a difference. The group receiving adjuvant radiotherapy showed almost 2-fold higher 5-year survival rates than the group without radiotherapy26). Intramedullary meningeal melanocytoma might show a similar prognosis to that of other meningeal melanocytomas since the origin of tumor cells are identical.
CONCLUSION
Intramedullary meningeal melanocytoma in the cervical spine is an extremely rare tumor. Here, we reported the case with a narrative literature review. MRI is the most frequently used diagnostic tool. However, the degree of sensitivity and specificity of MRI are insufficient to confirm intramedullary meningeal melanocytoma. Microscopic confirmation using immunohistochemical markers is an essential step for the final diagnosis. For the treatment of diseases, the most important aspect of prognosis is the complete resection of intramedullary meningeal melanocytoma. In addition, adjuvant radiotherapy should be considered even though complete resection has been performed. The possibility of a remnant tumor on the resection margin always exists even if it has been completely removed. Hence, adjuvant radiotherapy should not be omitted.
Notes
No potential conflict of interest relevant to this article was reported.

