AbstractAtypical teratoid/rhabdoid tumors (AT/RTs) are rare embryonal tumors. Herein, we present an unusual case of an AT/RT showing rapid regrowth to more than its original size approximately 1 month after gross total resection (GTR). A 22-year-old man presented with a severe headache. On magnetic resonance imaging (MRI), a tumor was found in the left cerebellopontine angle (2.8 × 3.7 × 3.7 cm; 38.3 cm3). GTR was performed, and biopsy confirmed AT/RT. On day 38 after surgery, a follow-up MRI due to abrupt mental deterioration to coma was performed, showing that the tumor had regrown to be even larger than its original size (3.6 × 3.5 × 5.3 cm; 66.78 cm3). After 1 month, the patient died. The median survival of AT/RT patients who undergo GTR is 20 months. In our case, the patient died much earlier than the previously reported overall survival duration after GTR. Therefore, physicians should always be wary of the possibility of rapid regrowth of AT/RTs when establishing the treatment plan.
INTRODUCTIONAtypical teratoid/rhabdoid tumors (AT/RTs) are rare embryonal tumors found in patients <3 years old15). There is no standard treatment regimen for AT/RT to date1). According to a study, 91%, 74%, and 42.4% of patients underwent surgery, chemotherapy, and radiation therapy respectively. The overall survival (OS) is 14.3 months (11.9-16.6 months), and the 5-year OS rate is 29.9%4). We present an unusual case of a patient that showed rapid regrowth at 1 month after gross total resection (GTR), and the patient died a month later.
CASE REPORTA 22-year-old man was admitted with a severe headache, left ear fullness with hypoacusis, dysphagia, left facial palsy (House-Brackmann grade III), left sternocleidomastoid muscle weakness (Medical Research Council [MRC] grade 4), right-side deviated uvula, decreased left anterior taste and left trapezius muscle weakness (MRC grade 4).
Magnetic resonance imaging (MRI) represented, a well-marginated, 2.8 × 3.7 × 3.7 cm (38.3 cm3) sized tumor in left cerebellopontine angle, which had both solid and cystic portions (Fig. 1A-C). Magnetic resonance spectroscopy showed a choline peak, decreased N‐acetyl aspartate (Cho/NAA: 2.01), and creatine, and increased lactate/lipid, suggesting malignant nature (Fig. 1D). The tumor was then removed via a retromastoid approach, but cerebellar intracranial hemorrhage occurred. So decompression by suboccipital craniectomy with navigation-assisted hematoma removal was performed and operation was successful (Fig. 2A-C). After GTR, the patient’s gag reflex mildly decreased, but the headache improved, and no additional neurologic symptoms were observed. And AT/RT was histologically confirmed (Fig. 3A-G).
While planning the treatment regimen including chemotherapy and radiation therapy with oncologists, the patient experienced abrupt mental deterioration to comatose. On follow-up MRI, the tumor was found to have regrown to size 3.6 × 3.5 × 5.3 cm (66.78 cm3), which was even larger than its original size (Fig. 4A, B). This was only a month after the surgery. Hence chemotherapy and radiation therapy could not be considered. Subsequently, secondary tumor removal with external ventricular drainage was performed because of obstructive hydrocephalus. However, hypoxic brain injury progressed with severe brain swelling, and the patient died 1 month after the event.
DISCUSSIONAT/RTs mainly occur in children under 3 years of age and are very rare embryonal tumors, accounting for 1% to 2% of all pediatric central nervous system tumors, classified as World Health Organization grade IV11,16). They commonly occur in the posterior fossa in children; however, they manifest supratentorially in adults17). According to Ostrom et al.12), incidence rates do not significantly differ by gender, race, or ethnicity. However, age has an effect on incidence. The incidence was highest at <1 year, and it decreased with age12). The median age at the time of diagnosis was 37 months14). Patients whose ages were less than 6 months had the worst prognosis14). Moreover, it is known that the prognosis for AT/RT is better in childhood than in adulthood2).
In diagnosing AR/RTs, there are key genes in addition to biopsy and imaging studies: SMARCB1 and SMARCA410). Bi-allelic mutations in SMARCB1 (INI1) at 22q11.2 are common, whereas SMARCA4 (BRG1) mutation at 19p13.2 is rare16). The SMARCB1 gene acts as a tumor suppressor gene and inactivation of both copies of this gene causes loss of protein expression in the nucleus in immunohistochemistry (IHC)13). These genes affect the prognosis. The age of onset of SMARCA4 mutated AT/RT is lower than that of SMARCB1 mutated AT/RT, and the survival period is 3 months. However, survival period of SMARCB1 mutated AT/RT is approximately 24 months6,8).
There is no standard regimen for AT/RT treatment; therefore, radiation therapy, chemotherapy, and surgical resection have been attempted7). It usually takes approximately 50 days to start chemotherapy3). Furthermore, radiation therapy takes an average of 93 days4). Table 1 summarizes some articles on AT/RT OS.
In addition, many studies on the survival period and rate of AT/RTs related to therapeutic modality. The AT/RT postoperative median survival is 20 months, and the disease-free survival is 14 months7). Lafay-Cousin et al.9) reported event-free survival of 14 months and median survival of 20 months in 20 patients who underwent GTR9). Schrey et al.14) demonstrated that the median recurrence-free survival and OS of patients who received radiotherapy were statistically higher than that of patients who did not receive radiotherapy 12.1 (5.216-7.034) and 23 (12.39-17.61) vs. 4 (3.314-4.686) and 10 (7.803-12.197), respectively14). According to Fossey et al.5), high-dose chemotherapy exhibits better outcomes than conventional chemotherapy (5-year survival rate of 52.0% and 10.7%, respectively5).
In our case, it was an adult-onset AT/RT, and the surgery was successful. Although genetic testing was not performed, SMARCB1 mutation was suspected based on IHC results. However, within 1 month after GTR, the tumor regrew to approximately twice the original size, and the patient died before chemotherapy or radiation therapy could be initiated. In our experience, AT/RT can progress rapidly even in patients with good prognosis factors. Physicians should always consider the possibility of rapid regrowth of AT/RT.
AcknowledgmentsWe would like to thank Dr. Se Hoon Kim, department of pathology, Yonsei University College of medicine for helping to read the biopsy.
Fig. 1.Preoperative magnetic resonance imaging. (A) T1, (B) T2, and (C) contrast-enhanced T1. A well-marginated, 2.8 × 3.7 × 3.7 cm (38.3 cm3) tumor in the left cerebellopontine angle, which had both solid and cystic portions. (D) Magnetic resonance spectroscopy shows a choline (Cho) peak, decreased N-acetyl aspartate (NAA) (Cho/NAA: 2.01) and creatinine, and increased lactate/lipid levels. 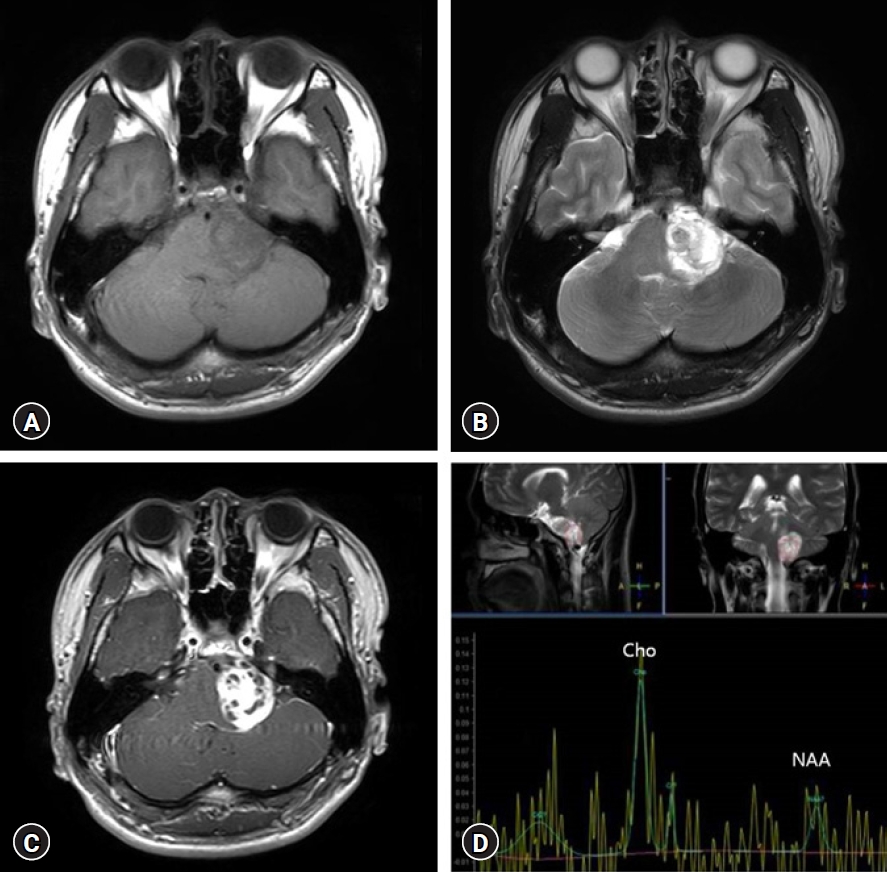
Fig. 2.Postoperative magnetic resonance imaging (MRI). (A) T1, (B) T2, and (C) contrast-enhanced T1. Postoperative MRI on day 1 after surgery. The tumor had been successfully removed. 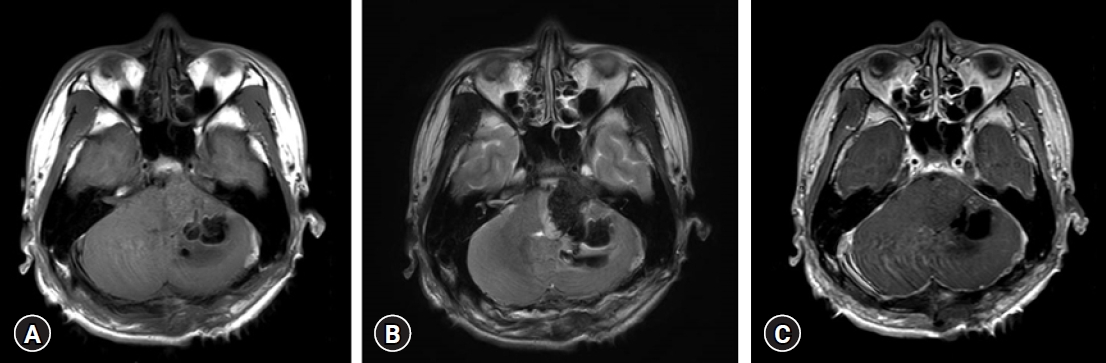
Fig. 3.Histology and immunohistochemistry. Histology of the atypical teratoid rhabdoid tumor shows areas with a rhabdoid phenotype, containing rhabdoid cells with eccentric nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (hematoxylin and eosin stain) (A), primitive undifferentiated components (B), mesenchymal components with spindle cells and collagen deposition (C), and reticulin surrounding the tumor cells (D). Characteristic immunohistochemical expression patterns reveal partial loss of integrase interactor 1 (E), negative cytokeratin (F), and strongly positive Brahma-related gene-1 cells (G). 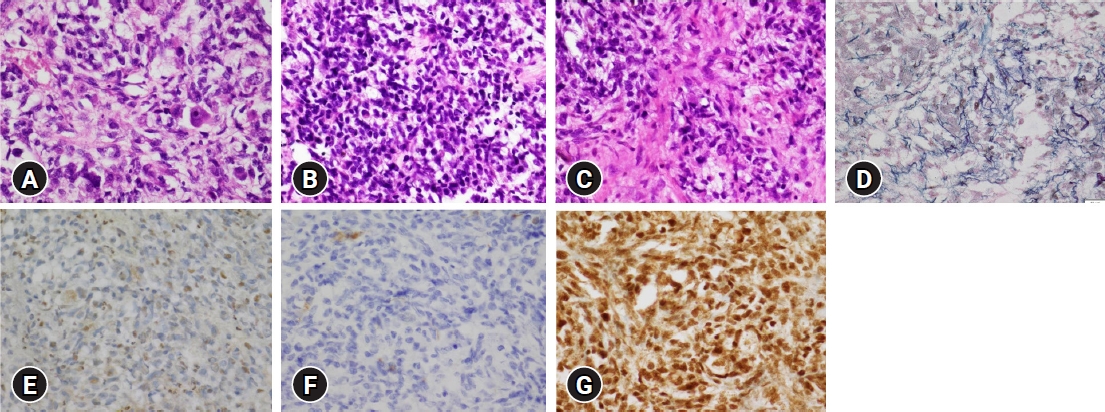
Fig. 4.Postoperative magnetic resonance imaging at 1 month after surgery. (A) T1-weighted, (B) T2-weighted. The tumor re-grew to 3.6 × 3.5 × 5.3 cm (66.78 cm3). 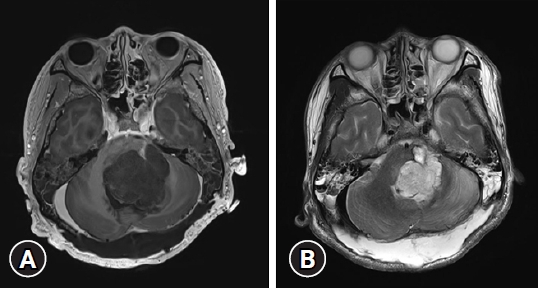
Table 1.Previously published articles about overall survival in atypical teratoid rhabdoid tumors
REFERENCES1. Athale UH, Duckworth J, Odame I, Barr R: Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol 31:651-663, 2009
2. Biswas A, Kashyap L, Kakkar A, Sarkar C, Julka PK: Atypical teratoid/rhabdoid tumors: challenges and search for solutions. Cancer Manag Res 8:115-125, 2016
3. Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, et al.: Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 27:385-389, 2009
4. Fischer-Valuck BW, Chen I, Srivastava AJ, Floberg JM, Rao YJ, King AA, et al.: Assessment of the treatment approach and survival outcomes in a modern cohort of patients with atypical teratoid rhabdoid tumors using the National Cancer Database. Cancer 123:682-687, 2017
5. Fossey M, Li H, Afzal S, Carret AS, Eisenstat DD, Fleming A, et al.: Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol 132:155-162, 2017
6. Hasselblatt M, Nagel I, Oyen F, Bartelheim K, Russell RB, Schüller U, et al.: SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol 128:453-456, 2014
7. Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, et al.: Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol 22:2877-2884, 2004
8. Holdhof D, Johann PD, Spohn M, Bockmayr M, Safaei S, Joshi P, et al.: Atypical teratoid/rhabdoid tumors (ATRTs) with SMARCA4 mutation are molecularly distinct from SMARCB1-deficient cases. Acta Neuropathol 141:291-301, 2021
9. Lafay-Cousin L, Hawkins C, Carret AS, Johnston D, Zelcer S, Wilson B, et al.: Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer 48:353-359, 2012
10. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al.: The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231-1251, 2021
11. Nesvick CL, Nageswara Rao AA, Raghunathan A, Biegel JA, Daniels DJ: Case-based review: atypical teratoid/rhabdoid tumor. Neurooncol Pract 6:163-178, 2019
12. Ostrom QT, Chen Y, P MdB, Ondracek A, Farah P, Gittleman H, et al.: The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro Oncol 16:1392-1399, 2014
13. Roberts CW, Biegel JA: The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther 8:412-416, 2009
14. Schrey D, Carceller Lechón F, Malietzis G, Moreno L, Dufour C, Chi S, et al.: Multimodal therapy in children and adolescents with newly diagnosed atypical teratoid rhabdoid tumor: individual pooled data analysis and review of the literature. J Neurooncol 126:81-90, 2016
15. Tekautz TM, Fuller CE, Blaney S, Fouladi M, Broniscer A, Merchant TE, et al.: Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol 23:1491-1499, 2005
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||