INTRODUCTION
Spinal dumbbell-shaped tumors are located in the intervertebral foramina or dura mater and exhibit a dumbbell shape. Surgery to remove these tumors that are located in the cervical intervertebral foramina may damage nerve roots or lead to incomplete tumor resection, vertebral artery injury, or post-laminectomy kyphosis2). Therefore, the best treatments for asymptomatic patients with these tumors remain debated. In the case of intracranial schwannoma, spontaneous shrinkage has been reported in more than 21 studies11). there are only 2 cases reports of spontaneous shrinkage of the spinal schwannoma7,8). Both cases involved shrinkage due to cellular reactions or chemical reactions to drugs, but we will present regression that arose during simple follow-up. This is a case report of spontaneous shrinkage of a cervical dumbbell-shaped tumor referred to our institution between November 2015 and November 2017.
CASE REPORT
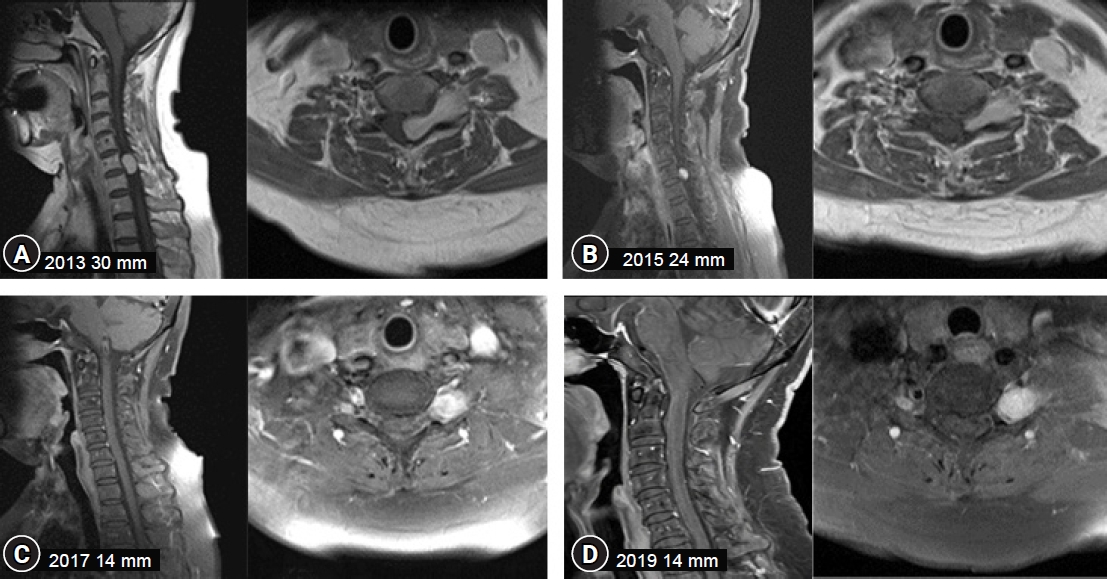
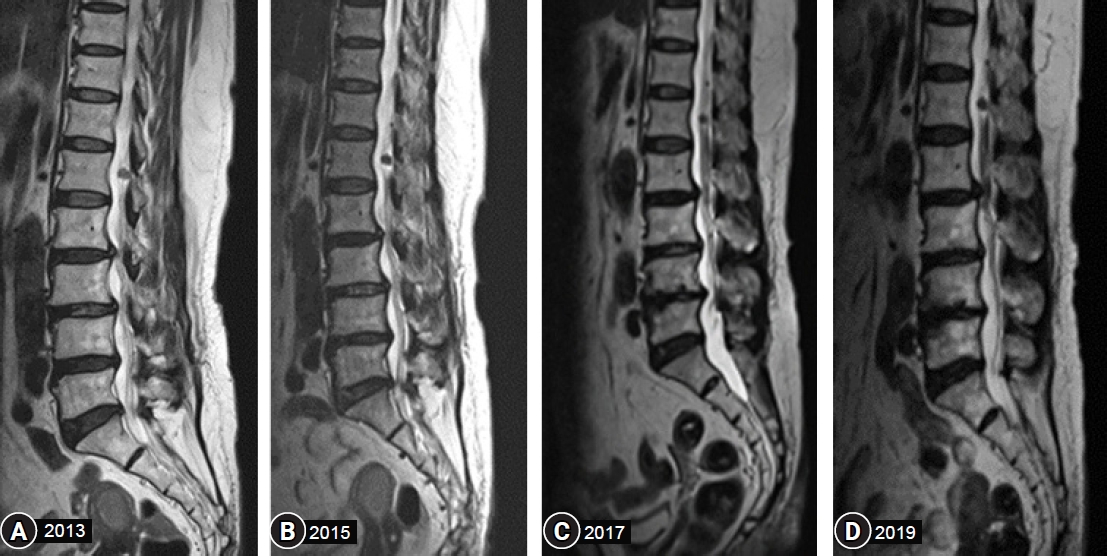
A 68-year-old female patient presented in 2013 with a progressive history of pain in the lower back and buttocks for the past 10 years. A radiological assessment that included spinal magnetic resonance imaging (MRI) was performed, multiple tiny neurogenic tumors around the lumbar spinal cord were observed, including a neurogenic tumor at the cervical 6/7 level. A 30-mm-sized tumor at the C6/7 level was compressing the spinal cord from the left and extended to the left C6/7 paravertebral space. There were few changes in tumor size in 2014, but an MRI obtained in November 2015 revealed that the dumbbell-shaped tumor had shrunk, leaving only the paravertebral component with a 24-mm maximal diameter from the axial view, and there were no new symptoms reported by the patient. Two years later, an MRI from November 2017 showed significant changes; the dumbbell-shaped tumor had decreased in size with a 14-mm maximal diameter (Fig. 1). In the annual follow-up, the tumor size did not decrease further from 2017 to 2019. The patient still had multiple suspicious schwannoma tumors in the lumbar region. There were no symptomatic changes or new symptoms after six years of follow-up visits (Fig. 2)
DISCUSSION
While generally uncommon overall, spinal schwannoma is the most common type of spinal extramedullary neurogenic tumor4,12). Spinal neurogenic tumors can be located anywhere in the intradural, epidural, and paravertebral space and can grow and compress the neural structures, causing pain or neurologic deficits. Symptoms may vary depending on the adjacent neural structure. The surgical approach should be selected according to the location of the lesion or adjacent neural structures. Although the surgical method varies depending on the location, surgical method may be complicated when it is located in both the extradural and paravertebral space1,16). Therefore, several classifications have been proposed accordingly10).
To resolve neurological symptoms and encourage regrowth, total gross removal is preferred over subtotal removal9). However, depending on the location’s complexity, a 2-stage procedure may be required for complex and large tumors; complications like a vertebral artery injury or post-laminectomy kyphosis have also been documented5,15). Therefore, due to its slow-growing, pathologically benign nature, surgical treatments in asymptomatic patients remain under debate3). Since there are many cases of benign characteristics of spinal neurogenic tumor, previous authors have suggested that if the tumor size does not increase by 2.5% or more per year, it is not growing very quickly6). In these cases, they recommended serial follow-up monitoring.
In the case of vestibular schwannoma (VS) of World Health Organization Grade I, some patients have demonstrated natural shrinkage. Between 1988 and 2013, 21 studies11). reported shrinkage of these tumors that ranged from 1% to 29% during follow-up periods that lasted from 6 months-27 years. The degree of tumor shrinkage identified ranged from 5.38% to 100% during the same follow-up. In contrast, few reports have described the spontaneous regression of a spinal cord dumbbell-shaped schwannoma. Ito et al.7) reported spontaneous regression after intramural hemorrhaging, and Kunnel Jomon et al.8) observed multiple myeloma diagnosed after the tumor’s discovery that was treated with pomalidomide and then regressed (Table 1).
In patients affected by neurofibromatosis type 2 (NF2) with bilateral VS, the gene responsible for NF2 is localized on chromosome 2214). However, there is currently no known genetic correlation with spontaneous shrinkage. von Eckardstein et al.13) described two patients with NF2 who underwent unilateral resection of VSs and demonstrated regression of the contralateral medium-sized VSs during follow-up periods of four and nine years, respectively. To our knowledge, no large series have described spontaneous shrinkage of VSs in patients with NF2. A multicenter study of 56 NF2 patients with 84 VSs reported 16 tumors out of 84 that regressed with a range of shrinkage between 1 and 7 mm during an average follow-up length of 51.3 months. Although genetic testing was not performed for NF2 in the present case, the shrinkage may be even greater in NF2 patients.
The principle of spontaneous tumor shrinkage is unclear, although there are a few theoretical conjectures in the literature13,14). Factors such as common genetics, environmental factors, and lifestyle influences are expected to affect this behavior. A reduction in vascular supply is the most promising hypothesis to date, and it is presumed to cause ischemic necrosis and fibrosis, resulting in spontaneous shrinkage of these tumors. A vascular genesis theory has been proposed that involves ischemic necrosis of the tumor caused by intra-tumorous thrombosis and subsequent fibrosis. The blood supply in the regional microenvironment where schwannomas grow may also contribute to spontaneous tumor shrinkage, especially in elderly patients. Tumor cell apoptosis or programmed cell death may also play a role in spontaneous tumor shrinkage. A comprehensive investigation of naturally occurring regression will be required to identify prognostic factors of spontaneous tumor shrinkage.
Our reported case indicates that simple follow-up visits can be helpful in the treatment of some tumors. It could make a chance that the surgical removal of schwannomas may be avoided if natural shrinkage of the tumor occurs.
CONCLUSION
The first choice of treatment for cervical schwannoma has typically been surgical resection. However, in this case, a tumor presumed to be a schwannoma naturally decreased in size. Clinicians should consider the potential for a spontaneous size reduction of cervical dumbell-shaped schwannomas.