Concurrence of Schwannoma and Meningioma Neighboring in the Lumbar Spine: A Case Report
Article information
Abstract
Multiple occurrences of spinal tumors are a rare phenomenon that usually results from underlying genetic diseases. Schwannoma and meningioma are well-known benign spinal tumors commonly found in the intradural extramedullary (IDEM) space with different cellular origins. We present an extremely rare case of neighboring concurrence of meningioma and schwannoma in the lumbar spine in a patient without an underlying genetic disease. Pathological studies regarding the concurrent IDEM tumors are demonstrated, along with a literature review.
INTRODUCTION
Schwannoma and meningioma are well-known commonly found benign spinal tumors in the intradural extramedullary (IDEM) space, each usually arising from Schwann cells and arachnoid cap cells in the meninges12,14). They comprise 80% to 90% of IDEM tumors, and sometimes preoperative radiological differentiation is necessary due to the possible difference in the surgical approach and removal technique. Occurrence of multiple spinal tumors is rare, which usually occurs related to genetic disorders like neurofibromatosis (NF) and von Hippel-Lindau (VHL) disease12). We report a very rare case of neighboring concurrence of meningioma and schwannoma in a patient without underlying genetic disease.
CASE REPORT
A 90-year-old man with a history of bladder cancer complained of pain for two years in both lower legs and progression of gait disturbance. In neurological examination, a symmetrical Medical Research Council grade 4 weakness was observed in both lower limbs but sensory change was not clear despite the pain complaint. There was no medical or familial history, and physical examination and radiological examination of brain and abdomen showed no evidence of genetic disorders such as NF of VHL. In image studies, T2-weighted magnetic resonance (MR) image demonstrated a coexistence of 2.4 × 1.2 × 1.6-cm sized heterogeneous low signal mass in L1 level and 1.5 × 2 × 1.2-cm sized homogenous low signal mass in L2 level each suggesting two different pathological masses, causing severe spinal stenosis in L1, L2 level. The tumors were located in the IDEM space, and the mass in L1 level had a more hyperintense signal than the mass in L2 level in T2-weighted image (WI).
On gadolinium (Gd) contrast administration, the tumor in the L1 level was heterogeneously well enhanced with a clear margin. The tumor in the L2 spinal level was homogenously enhanced and had the wide dural base. Intradural mass in the L1 level were more prominently Gd enhanced (Fig. 1). On computed tomography images, coarse calcification was observed in the mass of L2 level.
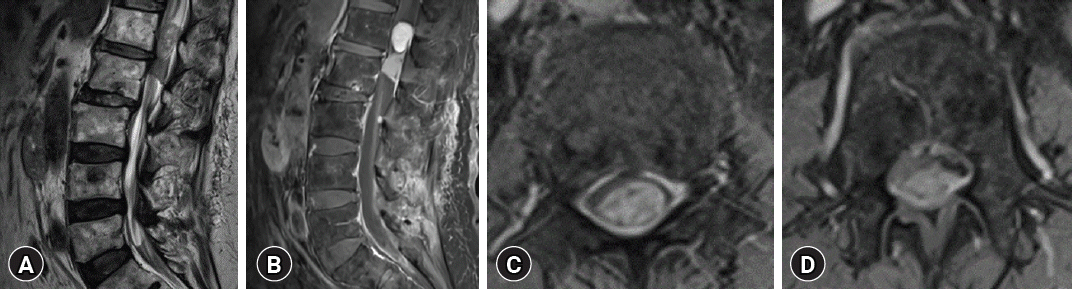
Magnetic resonance image of the lumbar spine. The 2 neighboring intradural extramedullary tumors were found at the L1-2 spinal level. The 2.4 × 1.2 × 1.6 cm mass at L1 showed a heterogeneous signal, and the second mass, which measured 1.5 × 2 × 1.2 cm, had a relatively homogeneous signal on T2-weighted imaging (A). Both tumors were well-enhanced on contrast enhancement and had clear demarcation (B). The upper mass was more prominently enhanced, whereas the lower mass had a wide dural base attachment (C, D).
1. Operation Procedure
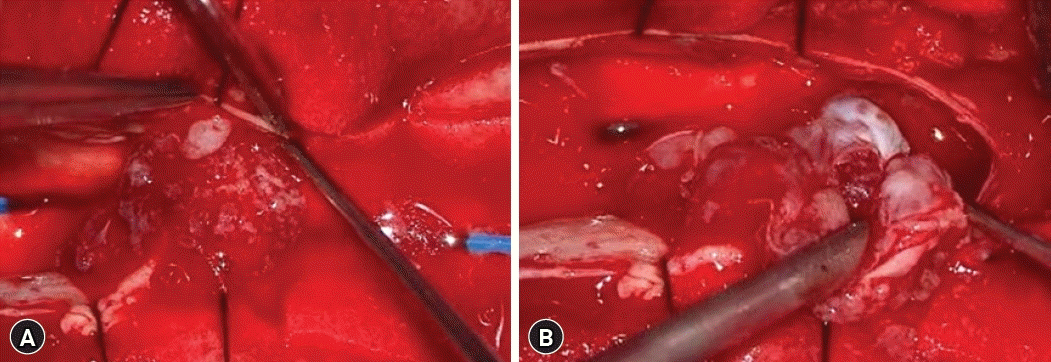
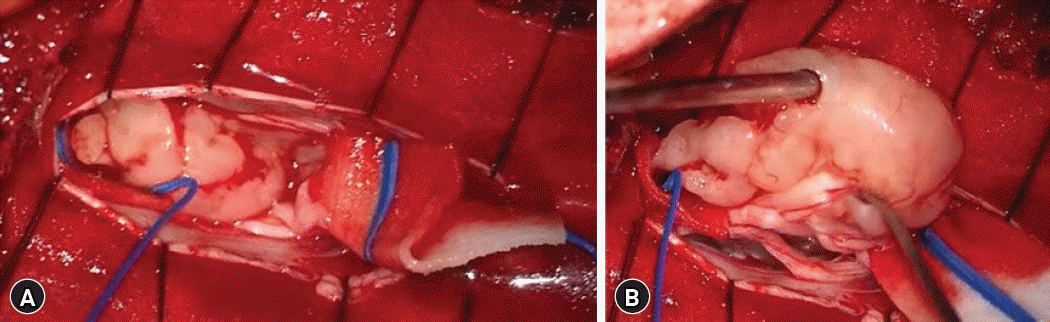
With the impression of IDEM tumors, the surgical approach was done via a T12-L1-2 laminoplastic-laminectomy in a prone position. Two intradural tumor at consecutive level was observed, each with distinctive gross characteristics. Tumors in L1, L2 level were neighboring but had different features without continuity. A whitish solid movable homogenous mass was observed in the L1 level, encapsulated and well-circumscribed mass was attached to the spinal nerve root. The mass was carefully dissected from spinal nerve root and en-bloc removal of the tumor was done without nerve root sacrifice (Fig. 2). Whereas the tumor in the L2 level has a pinkish, soft fragile configuration and this partially calcified lesion was attached to dorsal spinal dura. The mass was dissected from adjacent structures and piecemeal resection was done. Remnant tumor adhered to dorsal dura was completely coagulated using bipolar coagulator. Dural incision was carefully sutured in a water-tight manner (Fig. 3).
(A) A mass at the L1 level. (B) A whitish solid mass attached to the spinal nerve root was totally removed.
2. Pathologic Findings
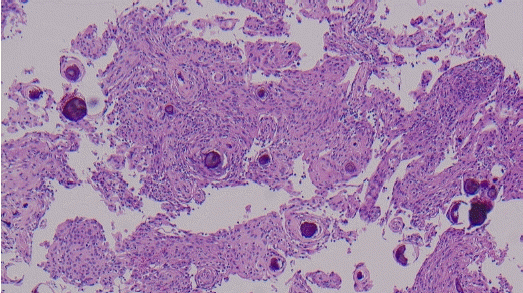
Intradural tumor in the L1 level showed proliferation of palisading spindle-shaped cells surrounding anuclear zones forming verocay body featuring cellular component of Antoni A and an acellular component of Antoni B. It also showed diffuse positive immunoreactivity in S100 stain. These characteristic findings are consistent with a diagnosis of schwannoma (Fig. 4.).
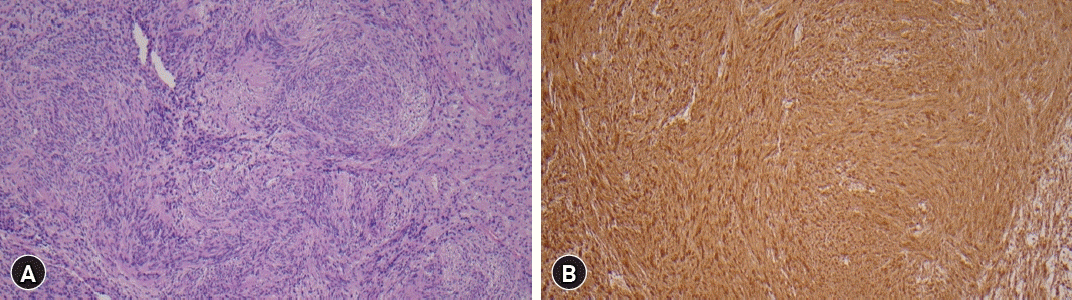
Histopathological findings of the tumor at the L1 level. (A) Spindle-shaped tumor cells with Verocay bodies were observed (hematoxylin and eosin stain, ×150 magnification). (B) Strong positive immunoreactivity was found in S100 staining, which is typical for schwannoma (S100 stain, ×100 magnification).
The tumor in the L2 level showed proliferation of spindle cells and multiple whirling or psammomatous features of meningothelial cells in hematoxylin and eosin (H & E) stained image, which lead to the diagnosis of a transitional cell type meningioma (Fig. 5).
DISCUSSION
Schwannoma and meningioma comprise 30% and 25% respectively of all spinal cord tumors6,12). The tumors have discrete histology and pathophysiology.
Schwannoma may occur at intradural, extradural space or with both intradural and extradural components. It usually occurs in the Schwann cell of the nerve roots. The 70% of intraspinal schwannomas are known to occur in the sensory roots 20% are in the motor roots, and the rest involve both sensory and motor roots.
Meningioma originates from arachnoid cap cells and may occur from anywhere in central nervous system (CNS) where dura is present. Spinal meningiomas account for 12% of all meningiomas and 80% of the spinal meningiomas are present at the thoracic level and 15% are found in cervical area10,12).
Sometimes, differentiating schwannoma and meningioma may be difficult with radiological study alone. Schwannomas usually show low signal intensity on T1-WI and high or mixed signal intensity in T2-WI, and well Gd enhanced. Irregular enhancement and encapsulated mass with ring-like pattern located eccentrically on peripheral nerves or spinal nerve roots are also usual characteristics1,10,17). Whereas, meningiomas are iso to low signal intensity in T1-WI and iso to high signal intensity in T2-WI which may be similar to schwannoma, but homogenous contrast enhancement on the attached dura base is characteristic (dural tail sign)1,6,10). Meningioma may have calcified or ossified part which is extremely rare in schwannoma5,11).
Regarding the anatomical characteristics of each of these tumors’ origin, schwannomas are usually observed from dorsal nerve roots located intra- and extra-dural space and may have a dumbbell shape18). Meningiomas are usually attached to dura and limited to the IDEM space. But it is not always possible to distinguish these tumors by identifying its origin with radiological findings alone in every case.
The occurrence of multiple primary spinal cord tumors is a very rare case that is not commonly observed even under conditions of NF7,9,18). There are three types of NF, NF-1, NF-2, and a rare third type of schwannomatois16). NF-1 is an autosomal dominant disorder caused by alteration of NF-1 gene located in 17q11.2, a tumor suppressor gene3,9,16). Mutation of NF-1 gene results in the diminished effect of tumor suppression, Ras gene hyperactivation and upregulation of mTOR and ERK pathways provoking various benign and malignant conditions such as café au lait spots, neurofibromas, pheochromocytomas, pilocytic astrocytomas, and malignant peripheral nerve sheath tumors3,9). Multiple spinal schwannomas or neurofibromas can be observed in patients with NF-1 gene mutation. NF-2 is an autosomal dominant hereditary disease caused by alteration in tumor suppressor gene of NF-2 on chromosome 22q12.2 which encodes amino acids called Merlin, which involves in stabilizing the membrane cytoskeleton interface by inhibiting signals involving PI3kinase/Akt, Raf/MEK/ERK, and mTOR signaling pathways2). Alteration of this tumor suppressor gene results in various central and peripheral nervous tumors such as vestibular schwannoma, peripheral schwannoma, intracranial or spinal meningioma, and ependymoma2,7).
Another condition vulnerable to tumor development in spinal cord is VHL disease, which is an autosomal dominant disease related to germline mutations in the VHL gene, which acts as a tumor suppressor gene located in chromosome 39,13,15). CNS hemangioblastoma (HBL) has been found in 60% to 80% of patients with VHL disease. Spinal cord HBL accounts for 13% to 50% of VHL-associated HBL in the CNS9). HBL is usually solitary, but it is often multiple in VHL disease8,15,20). However, multiple spinal schwannoma and meningioma are not related to VHL gene mutation.
The mechanism of occurrence of different tumors in the same location is not well explained. Occurrence of multiple tumors can be assorted as a (1) mixed tumor in which characteristics of multiple tumors are found in one tumor; and a (2) concurrent tumor in which different tumors are found at the same time. The second category is compatible with the present case because occurrence of distinct tumors of schwannoma and meningioma simultaneously4,12,14,17). Independent coincidental development of different tumors in same anatomical site is the first explanation of the case and it accounts for the rarity of the incidence12).
Second hypothesis is the presence of the first tumor causing changes in surround microenvironment, facilitating the development of the second primary tumor4,12,14). Vascular endothelial growth factor was suggested as a related factor, as it is known to be involved in angiogenesis and tumor expression12). An upregulation of hypoxia-inducible factor in VHL may also be another example of multiple occurrences of tumor that causes microenvironmental changes. Size differences in tumors may be the evidence of the theory. The larger tumor is the preexisting tumor and the smaller tumor is the secondary tumor that occurs later in multiple tumor occurrences.
Another hypothesis is that common mesenchymal progenitor cells may separately differentiate into schwannoma and meningioma at the same spinal level. Schwannoma and meningioma are each thought to arise from Schwann cell and arachnoid cap cell, however there are theories that these cells are results of multidirectional growth of same mesenchymal cells17). Further evidences must be gathered to prove this hypothesis of multiple occurrences of spinal cord tumors.
A similar report to our case was rarely found in the literature. Matsuda et el.12) reported two cases of concurrent extradural schwannoma and intradural meningioma at the same level in the upper cervical level. Both cases showed tumors composed of intradural and extradural portions so that the tumor appeared as a dumbbell-shape. Both tumors were confirmed as extradural schwannoma with intradural meningioma by pathological study.
Porčnik et el.19) also reported a very similar case to our present case in 2020. They reported a case 59-year-old male without genetic disease history with single tumor-mimicking IDEM meningioma and schwannoma in L1-2 level. The tumors were neighboring and had slightly different signal intensity in MR imaging, and pathological diagnosis were confirmed with operative removal.
CONCLUSION
We present a remarkably rare case of concurrent spinal schwannoma and meningioma within lumbar spine. This unique co-occurrence of two distinct neural tumors in such proximity is an exceptional and infrequently documented phenomenon in the literature. Our report underscores the importance of comprehensive diagnostic evaluation and precise surgical planning when encountering complex spinal lesions, particularly when faced with atypical presentations. Further research and exploration into the underlying mechanisms of such coexistence may provide valuable insights into the etiology and management of spinal neoplasms.
Notes
No potential conflict of interest relevant to this article was reported.